
A structured tool utilized by individuals overseeing clinical trials to ensure comprehensive education for nursing staff involved in research activities. This tool typically outlines essential training components, competency assessments, and documentation requirements... Read more »

A standardized instrument assists clinical trial nurses working in patients’ residences to perform consistent and thorough data collection. This tool ensures that essential elements of the research protocol are addressed during each... Read more »

A structured tool designed for use by individuals overseeing research studies, it ensures that nurses involved in data collection and patient care are adequately prepared in the specific guidelines and procedures of... Read more »

A structured inventory ensuring competency of clinical research personnel is essential before commencing a new research investigation. This inventory systematically verifies that study nurses possess the necessary knowledge, skills, and understanding of... Read more »
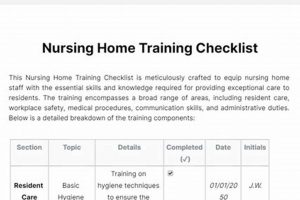
The instrument designed to ensure study nurses are adequately prepared to implement revised research procedures comprises a structured list of competencies and tasks. This inventory verifies comprehension of new guidelines, standard operating... Read more »

A structured document outlining the required training for personnel involved in the initial phases of a research project is essential. This document ensures that all staff members possess the necessary knowledge and... Read more »
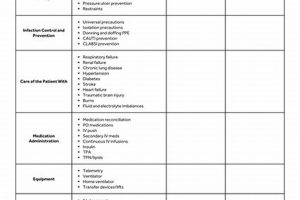
A document designed to verify the competence of research personnel in executing protocols and procedures unique to a particular clinical trial. It itemizes tasks, knowledge areas, and skills requiring demonstration of proficiency... Read more »
A comprehensive document ensuring personnel involved in research studies are adequately prepared for their roles. This tool details required training elements tailored to the unique protocol of a particular investigation. For instance,... Read more »


