
The phrase identifies evaluations of medical interventions or products conducted by a specific organization. These assessments adhere to established scientific protocols and regulatory guidelines to determine the safety and efficacy of the... Read more »
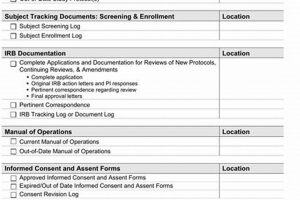
A structured tool designed to ensure study nurses are adequately prepared for the implementation of a novel research plan. It systematically verifies comprehension of the updated research procedures, data collection methods, ethical... Read more »

Research endeavors conducted within a specific metropolitan area in South Florida, involving human participants, aim to evaluate the safety and efficacy of novel medical interventions. These investigations may encompass pharmaceuticals, medical devices,... Read more »

Compensation for professionals overseeing the lifecycle of clinical trials is a multifaceted subject. This remuneration reflects experience, geographic location, employer type (pharmaceutical company, contract research organization, academic institution), and the complexity of... Read more »

A standardized document used by clinical trial study nurses during in-home patient visits facilitates the collection of consistent and accurate data. This tool often incorporates pre-defined fields for recording vital signs, medication... Read more »
Resources designed to aid individuals preparing for roles in managing clinical trials and related projects, available without cost, and structured in a checklist format for efficient study planning. These resources typically outline... Read more »

A structured document designed to guide study nurses during in-home assessments conducted as part of decentralized clinical trials (DCTs). It provides a standardized framework for data collection, patient interaction, and safety monitoring... Read more »

A structured tool utilized by individuals overseeing clinical trials to ensure comprehensive education for nursing staff involved in research activities. This tool typically outlines essential training components, competency assessments, and documentation requirements... Read more »
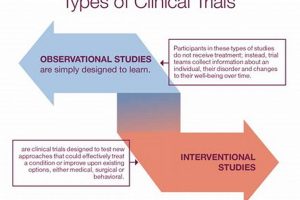
A systematic investigation designed to evaluate the effects of a medical, surgical, or behavioral intervention is frequently undertaken. These research endeavors rigorously examine interventions, often comparing them against a standard treatment or... Read more »

A structured tool designed for use by individuals overseeing research studies, it ensures that nurses involved in data collection and patient care are adequately prepared in the specific guidelines and procedures of... Read more »


