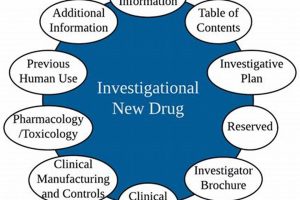
Maintaining documentation related to clinical trials involving experimental medications is a regulatory mandate. This requirement, enforced by the Food and Drug Administration (FDA), ensures the availability of comprehensive data concerning investigational drugs... Read more »

