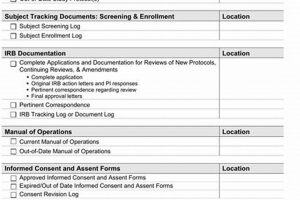
A structured tool designed to ensure study nurses are adequately prepared for the implementation of a novel research plan. It systematically verifies comprehension of the updated research procedures, data collection methods, ethical... Read more »
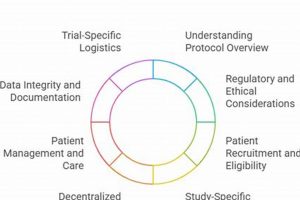
The specifications for instruction related to research procedures for nursing professionals engaged in clinical research are formalized guidelines. These specifications ensure that nurses possess the knowledge and skills necessary to execute research... Read more »

A structured inventory ensuring competency of clinical research personnel is essential before commencing a new research investigation. This inventory systematically verifies that study nurses possess the necessary knowledge, skills, and understanding of... Read more »

A structured document used to verify the comprehensive education and preparation of research nurses, ensuring adherence to established guidelines and standard operating procedures prior to participating in clinical trials. It serves as... Read more »
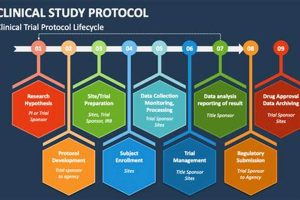
The systematic education of study nurses regarding the standardized guidelines and procedures governing clinical research endeavors is a critical component of ensuring data integrity and participant safety. This specialized instruction encompasses a... Read more »


