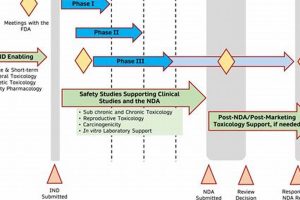
Research efforts designed to facilitate the submission and approval of Investigational New Drug (IND) applications are crucial in the pharmaceutical and biotechnology industries. These investigations encompass a range of pre-clinical assessments, including... Read more »

